
[Information for this page was supplied by Maria Costa, and was edited for the site by John Cheeseman.]
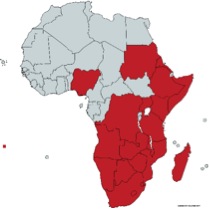
Xerophyta is a monocotyledonous genus from the family Velloziaceae. It is usually considered the only African genus in the family, although Talbotia (with one species, Talbotia elegans aka Xerophyta elegans), is sometimes considered to be a separate genus. Forty-five species of Xerophyta are recognized in Africa (see map), one species is known to occur on the Arabian Peninsula, and 25 species are present in Madagascar 1. The name comes from Ancient Greek ξηρός (xeros, “dry”) and φυτά (phutá), plural of φυτόν (phutón, “plant”). The closest relatives of Xerophytas are the South-American Vellozia. The distribution of the family Velloziaceae suggests a Gondwanan origin and that the split between African and South American species corresponds to the splitting of the two continents, around 100 Mya. 2
Like other monocots, Xerophyta spp. are poikilochlorophyllous, i.e. they lose their chlorophyll during desiccation. X. viscosa, the only species with a sequenced genome, is a chasmophyte. It is also self-incompatible and thus shows a high degree of heterozygosity. In contrast, X. humilis, for which transcriptome resources are available, is a non-chasmophyte.
Available resources for X. viscosa
To date, the only species of Xerophyta whose genome has been sequences is X. viscosa. 5 Despite being octoploid, its genome size is only ~295.5 Mb (haploid), distributed over 48 small chromosomes. The percentage of orphan or taxon restricted genes is lower than expected for a eukaryotic genome, only 5.4%. At 18%, the fraction of the genome occupied by transposable elements (TE) is also low. Based on this and the fact that other DT genomes are 40-75% TE, Costa et al.5 concluded that the proportion of TEs in a genome does not appear to be related to DT in general. Based on mapping of orthologous groups, it does not appear that, overall, X. viscosa has undergone extensive expansions or contractions of gene families. The low overlap of those that do occur with expanded or contracted gene families in other DT plants has been taken to suggest different genetic architectures underlying the resurrection phenotype in different species.
The X. viscosa genome is available as NCBI accession PRJNA295811.
Available resources for X. humilis
NCBI Bioproject resources are also available for the desiccation-responding transcriptomes of X. humilis leaves and for leaves, roots and mature seeds. In the leaf project, mRNA transcript abundances were compared for 1709 cDNAs at 6 relative water contents (RWC) (100%, 80%, 60%, 40%, 20% and 5%RWC respectively). In the latter project, transcript abundance of 1709 X. humilis mRNAs were compared in desiccated (5% RWC) and hydrated (100% RWC) leaves and roots, and in mature seeds.
Acknowledgements – The developers thank Maria-Cecilia Costa (University of Cape Town, Mel Oliver (USDA/University of Missouri), Don Gaff (Monash University) and Dorothea Bartels (University of Bonn) for their input and assistance in developing the resurrection plants pages.
Bibliography
